Amid growing public concern over the rising number of failed treatments for malaria and typhoid fever across Africa, Nigeria’s food and drug regulatory agency has confirmed the troubling cause to be the circulation of substandard and falsified versions of two commonly used medicines.
Many patients have reported that common treatments are no longer effective, prompting investigations by the regulatory body, the National Agency for Food and Drug Administration and Control (NAFDAC) which has identified two such medications sold in Nigeria as dangerously below standard, further fueling fears over the safety and effectiveness of widely used drugs.
In an official alert issued during the week, NAFDAC has officially confirmed to the public the sale of substandard and falsified versions of Artemetrin DS and CIPROFIT 500 tablets in the country.
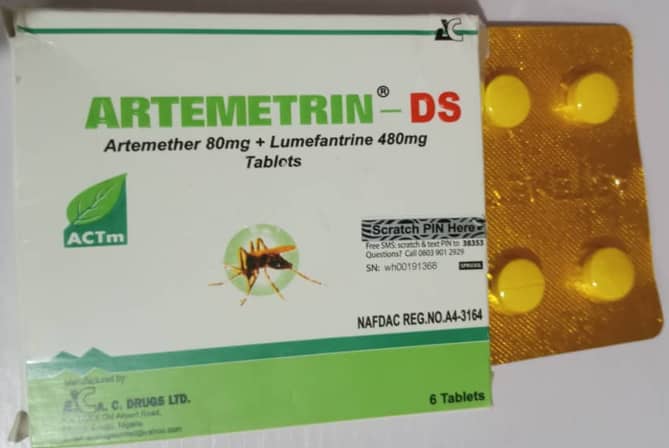
According to the statement by NAFDAC, the ARTEMETRIN DS (Artemether/Lumefantrine) tablet (80mg/480mg) is labeled as manufactured by A.C. DRUGS Ltd, Plot C5/C6 Old Airport Road, Emene, Enugu State, Nigeria.
Similarly, the CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) is labeled as manufactured by Impact Pharmaceutical Ltd, No. 33A/33B Standard Industrial Layout, Emene, Enugu State, Nigeria. Both products were subjected to Thin-Layer Chromatography (TLC) tests, and they showed significant irregularities, leading to further analysis at a WHO-prequalified laboratory. The High-Performance Liquid Chromatography (HPLC) results conducted by NAFDAC revealed that ARTEMETRIN DS contained only 59.2% Artemether and 71.2% Lumefantrine, well below the accepted range of 90–110%.
CIPROFIT 500, on its part was found to contain just 5.7% Ciprofloxacin, making it essentially ineffective.
Interestingly, both products were obtained from a “licensed vendor and wholesaler”, yet they do not appear in the NAFDAC registered product database. Moreover, the NAFDAC Registration Numbers printed on the packaging are fraudulent.
Sequel to these results, NAFDAC has urged the public to immediately stop the sale or use of these products and return any stock to the nearest NAFDAC office, requesting that anyone who has used either of these medications and is experiencing adverse effects should seek urgent medical attention from a qualified healthcare provider.
Meanwhile, healthcare professionals and consumers have been asked to report suspected cases of substandard or falsified medicines to NAFDAC via their hotline at 0800-162-3322 or by email at sf.alert@nafdac.gov.ng.