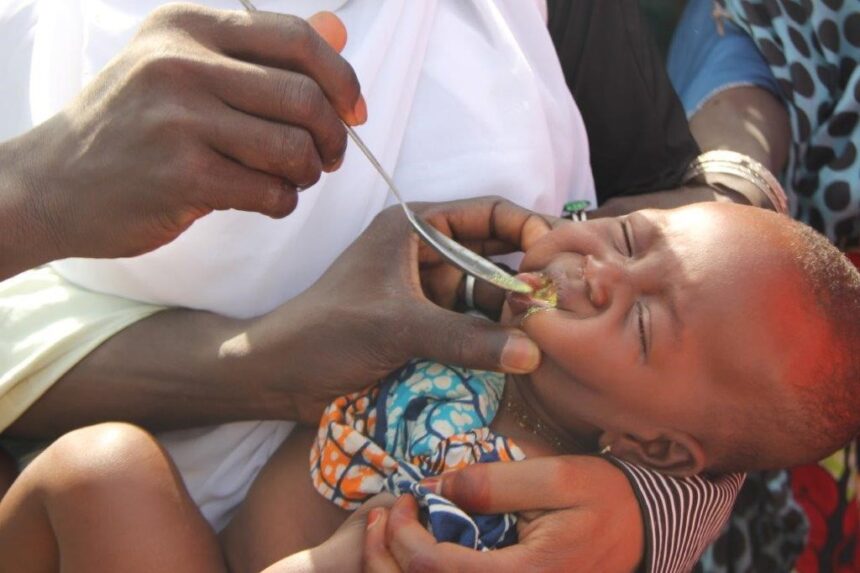
Eight African countries have undertaken the clinical trial of the world’s first malaria treatment specifically formulated for newborns.
The new formulation of artemether-lumefantrine, which is the first malaria treatment approved for infants weighing less than five kilograms has been found to dissolve easily in breast milk and has a sweet taste, was successfully tested in Nigeria, Burkina Faso, Côte d’Ivoire, Kenya, Malawi, Mozambique, Tanzania, and Uganda.
Malaria remains one of Africa’s deadliest diseases, with newborns and children under five at greatest risk. Nigeria alone accounts for nearly one-third of global malaria deaths, according to the World Health Organization (WHO). Beyond the tragic human toll, malaria drains billions of dollars annually from Africa’s economy through lost productivity, treatment costs, and pressure on fragile health systems.
Meanwhile, the Swiss health regulators have already cleared the infant-friendly drug. Nigeria and the other trial countries are expected to accelerate national approvals through the Swiss-backed marketing authorisation procedure for global health products.
Dr. Jean Kaseya, Director-General of the Africa Centres for Disease Control and Prevention (Africa CDC), described the approval as “a major step forward in the fight against malaria.” His principal adviser, Dr. Ngashi Ngongo, added that the success of the trial demonstrates Africa’s capacity to drive health innovations, noting that Nigeria’s contribution was central to the outcome.
Africa CDC said it would support governments to integrate the treatment into national health systems by updating guidelines, training frontline health workers, and ensuring access in rural and underserved communities where the malaria burden is heaviest.
This development aligns with Africa’s ambition to eliminate malaria by 2030, a target adopted by the African Union and reinforced in the WHO’s Global Technical Strategy for Malaria. For Nigeria, which launched its National Malaria Strategic Plan 2021–2025 with the aim of reducing malaria prevalence to less than 10 percent, the new drug offers a timely tool to protect the most vulnerable citizens.
The breakthrough was achieved through collaboration between Novartis and Medicines for Malaria Venture (MMV) under the PAMAfrica consortium, which focuses on advancing practical and affordable malaria treatments across the continent